© 2025 LaunchKU, All Rights Reserved
A big THANK YOU from all of us here at the Midwest Stem Cell Therapy Center! Because of your support, we are able to advance our work on several exciting adult stem cell research efforts!

We hope everyone’s new year is off to a great start. We here at the Midwest Stem Cell Therapy Center have hit the ground running for 2016! We thought we’d share with you another one of our research efforts…
Researchers at the University of Kansas Medical Center and the Center are working to find a therapy that would delay the progression of Amyotrophic Lateral Sclerosis (ALS). This effort is championed by Dr. Hiroshi Nishimune, Associate Professor, in collaboration with the Center and the KU Department of Neurology, led by Dr. Richard Barohn, an internationally known clinical scientist in the field of ALS research trials.
An essential part of this research includes mesenchymal stem cells cultivated by the Center. KU researchers are looking into how these stem cells can slow the progression of ALS. Dr. Nishimune is using an ALS mouse model to learn more about this terrible disease. Together, this team is making progress in understanding and ultimately treating ALS.
According to Dr. Nishimune, “One of the main obstacles of ALS is that we don’t yet know the etiology of the sporadic cases, which comprises about 90% of the ALS patients. If stem cells can at least delay its progression, patients will be able to enjoy a better quality of life.”
These initial mouse studies are critical to future studies with patients. Once we demonstrate stem cells developed by the Midwest Stem Cell Therapy Center can slow down ALS in mice, we can begin working towards stem cell research in ALS patients.
Thank you so much for supporting the Midwest Stem Cell Therapy Center this year. We are eager to continue our adult stem cell research in 2016 – stay tuned for exciting updates!

We’re excited to share that the Midwest Stem Cell Therapy Center, in collaboration with KU’s Department of Pharmacology, Toxicology & Therapeutics, has made great strides in one of its research areas, liver repair.
Data shows our adult stem cells, after being introduced to a damaged/diseased liver, help return the organ to normal function more quickly than if it were to heal on its own.
Why is this important?
This could be significant to the one in 10 Americans who have some form of liver damage/disease. The liver carries away waste, breaks down fat, stores energy and clears poisons from the blood. When it doesn’t function properly, serious problems arise. Our pre-clinical research shows the Center’s adult stem cells, which are developed here at KU Medical Center, help restore that lost function. While further research is being conducted, this preliminary research shows incredible potential.
Because of your support, those suffering from liver damage/disease may have hope. It is because of you that we are able to make progress in our other research efforts, including Graft-versus-Host Disease, a program being developed with input from the FDA.
Your support makes adult stem cell treatments a possibility!

The journey for a new drug – from the laboratory to the general public – is a long and complex one. Every approved drug here in the United States has gone through the Food and Drug Administration’s (FDA’s) rigorous evaluation process. Here at the Center, we are working on multiple research efforts that are at various phases in the drug/treatment development process!
Why is this process so important?
When you are prescribed a medication or treatment, you want to be assured it is both safe and effective. That’s where the FDA comes in. They have a process that ensures each new treatment or drug introduced to the public has been thoroughly tested for both safety and effectiveness. This process, although timely and expensive to the researching organization, is imperative to public safety.
What is the process?
Pre-Clinical
Conducted before human trials can begin.
Focused on developing data for the manufacturing process, methods to characterize the stem cells of interest, and animal safety and effectiveness.
Clinical Trial Phase I
Drug or treatment is first administered to humans.
Researchers test the treatment in a small group of people to evaluate its safety, determine a safe dosage range, and identify side effects.
Clinical Trial Phase II
Effectiveness tested in humans.
The treatment is given to a larger group of people (≤ 100) who have the condition to be treated to determine if it is effective against the disease target and to further evaluate its safety.
Clinical Trial Phase III
Safety and effectiveness are further assessed.
The treatment is administered to a large groups of people (typically > 400) to confirm its effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the FDA to determine if it can be marketed.
Clinical Phase IV
Conducted after the adult stem cell treatment has been marketed to gather additional information on the drug's effectiveness and safety in various populations.

The initial concept of regenerative medicine dates all the way back to 330 BC, when Aristotle observed that a lizard could grow back the lost tip of its tail. Slowly over time, humans have grown to understand regenerative medicine, and how it may change the way we treat diseases. It's been only relatively recently that adult (non-embryonic) stem cell therapy, a type of regenerative medicine, has gathered fast momentum.
The video shows key (not all) highlights in stem cell research, illustrating how quickly research has progressed over the last several decades. While researchers have come a long way, there are still many discoveries to be made. Here at the Midwest Stem Cell Therapy Center we are working with the appropriate regulatory agencies, including the FDA, to advance adult stem cell treatments.
We hope everyone had a wonderful Thanksgiving!
Here at the Center, we have several ongoing research collaborations. Below is a highlight of one of our research efforts.
Graft-versus-Host Disease, or GvHD, is a complication that can occur after a bone marrow or stem cell transplant. With GvHD, the newly transplanted donor cells attack the patient’s cells. It’s similar to when a patient’s body rejects a new organ transplant…except the roles are reversed.
GvHD happens as a result of the immunological cells (T-cells) that are in the bone marrow. When your body has an infection, it is your T-cells that detect the infection and fight it off. While bone marrow recipients are given immune-suppressing drugs to inhibit T-cell response, the donor T-cells may still detect the new host (the patient) as foreign. In the case of severe forms of GvHD, the immune response can be fatal. In fact, more than 90% of patients who contract GvHD that cannot be managed by immune-suppressing drugs will die within five years of diagnosis.
In partnership with the University of Kansas Cancer Center, the Midwest Stem Cell Therapy Center has developed stem cells isolated from Wharton’s Jelly (solid part of the umbilical cord) as a potential therapy to increase the survival rate of patients diagnosed with GvHD. In our experiments, these cells have been shown to inhibit T-cell growth, reducing the donated bone marrow’s immune response to the new host.
Next Steps…
The Center is currently working through the FDA’s Investigational New Drug (IND) program. Every drug or treatment that is FDA-approved has gone through this multi-phase program in order to prove their safety and effectiveness.
With the Center’s approach and with FDA approval, patients suffering from Graft-versus-Host Disease may have a greater chance of surviving this terrible disease.
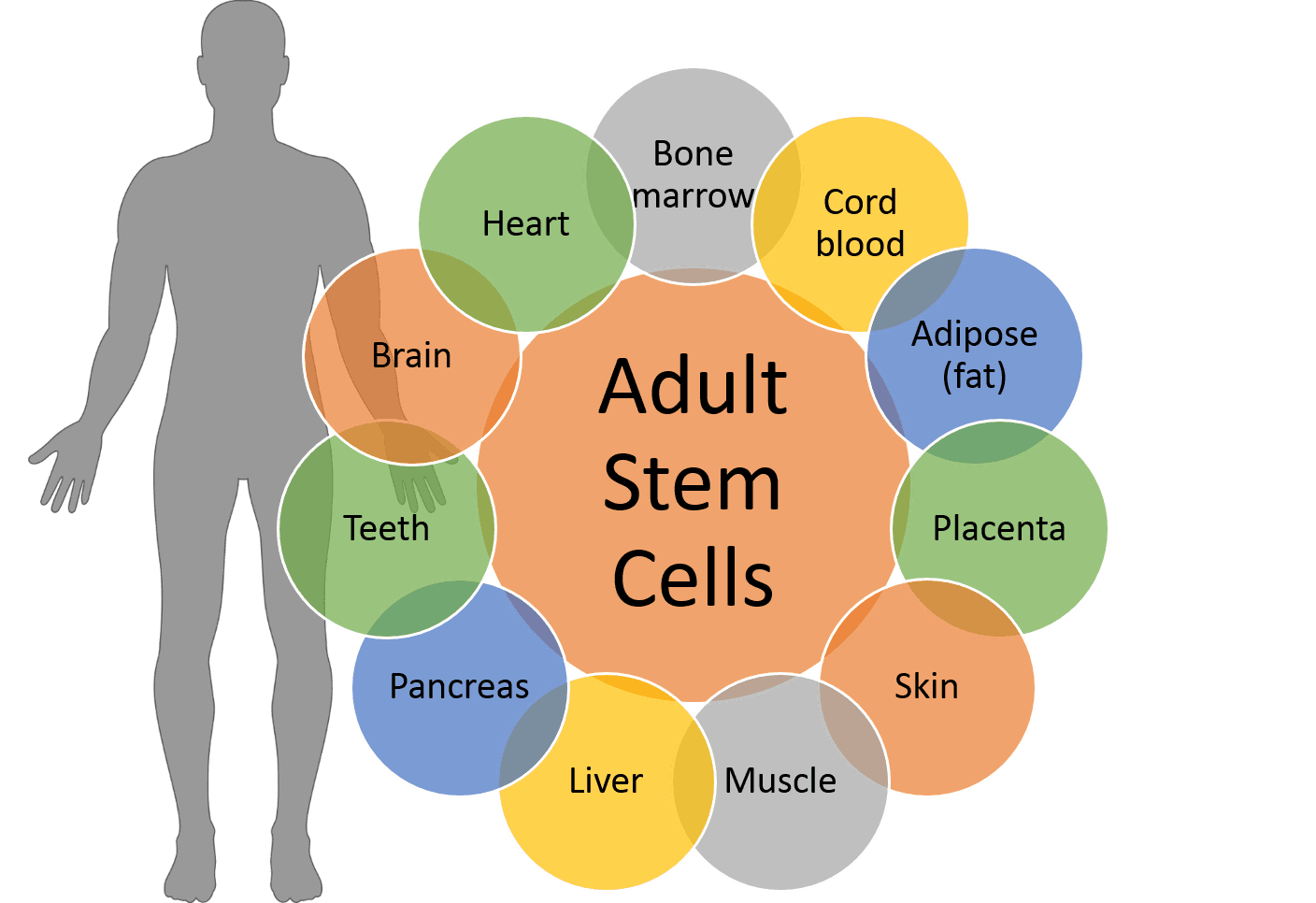
Adult stem cells have been found in most parts of the body, including fat, brain, bone marrow, blood vessels, skin, teeth and heart. A source particularly rich in stem cells is called Wharton’s Jelly, the solid part of the umbilical cord (which is usually discarded after birth). Researchers at the Midwest Stem Cell Therapy Center isolate stem cells from the Wharton’s Jelly and expand, or “grow”, the number of cells using our expansion system.
After growing them in large quantities – exceeding hundreds of millions of cells – researchers then manipulate them to produce specific cell types that can be used to treat a disease or condition. Stay tuned for our next update, which will cover one of our latest disease research efforts.
Have a happy Thanksgiving!
First, thanks to all of our donors so far! Thanks to you, we are that much closer to our $25,000 goal.
You’ve probably heard the term “stem cells” in the news, but there’s a lot of information out there and it can be confusing to sort through all of it. It’s first important to understand that, based on origin, there are two different kinds of stem cells:
Embryonic Stem Cells
Adult Stem Cells
The Midwest Stem Cell Therapy Center works exclusively with adult stem cells because we believe – and recent research shows – that they have great potential when it comes to treating diseases and other health conditions.
Adult (non-embryonic) stem cells are unspecialized or undifferentiated cells, which means they have yet to develop into a specific cell type. Found in most adult tissues, adult stem cells have two primary characteristics:
They are able to self-renew, i.e., they have the ability to go through numerous cycles of cell division while maintaining their undifferentiated state.
They have, as a group, unlimited potency, i.e., they have the capacity to grow into any of the body's more than 200 cell types.
Because an adult stem cell can renew itself, it acts as a “repair kit” in our bodies – repairing and regenerating tissue as needed.
Here at the Midwest Stem Cell Therapy Center, our researchers are collecting and expanding (growing) adult stem cells, and differentiating them to see how they can be used in a variety of diseases and conditions.
The picture shown here is that of a mesenchymal stem cell isolated from Wharton’s Jelly, a part of the umbilical cord. Adult stem cells typically exhibit this spindle or fibrobrast-like shape.

Purchases cell culture items needed to isolate stem cells from a tissue source
Allows us to isolate stem cells from one umbilical cord
Allows us to establish a “banked” cell source for future study
Allows us to harvest 60 million stem cells (we need 1200M stem cells)
Allows us to double the number of clinical grade stem cells available – we need 10 doublings per umbilical cord